When and when not do foods, based on the process used for their production, fall under the Novel Food Provisions? The answer to this question is found on this page.
Legal Framework:
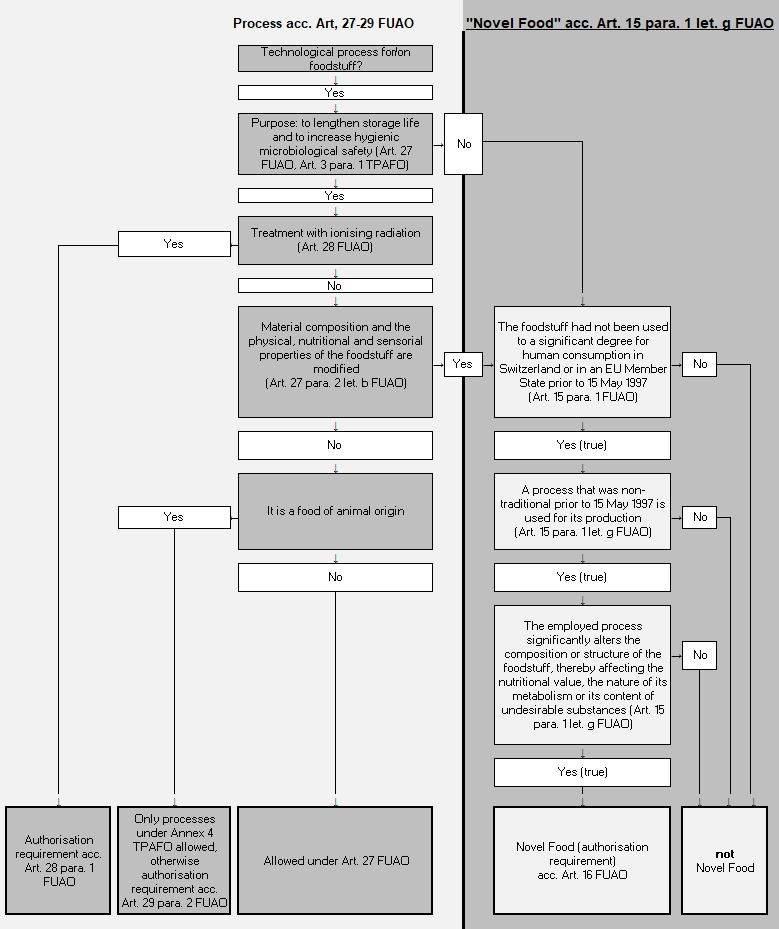
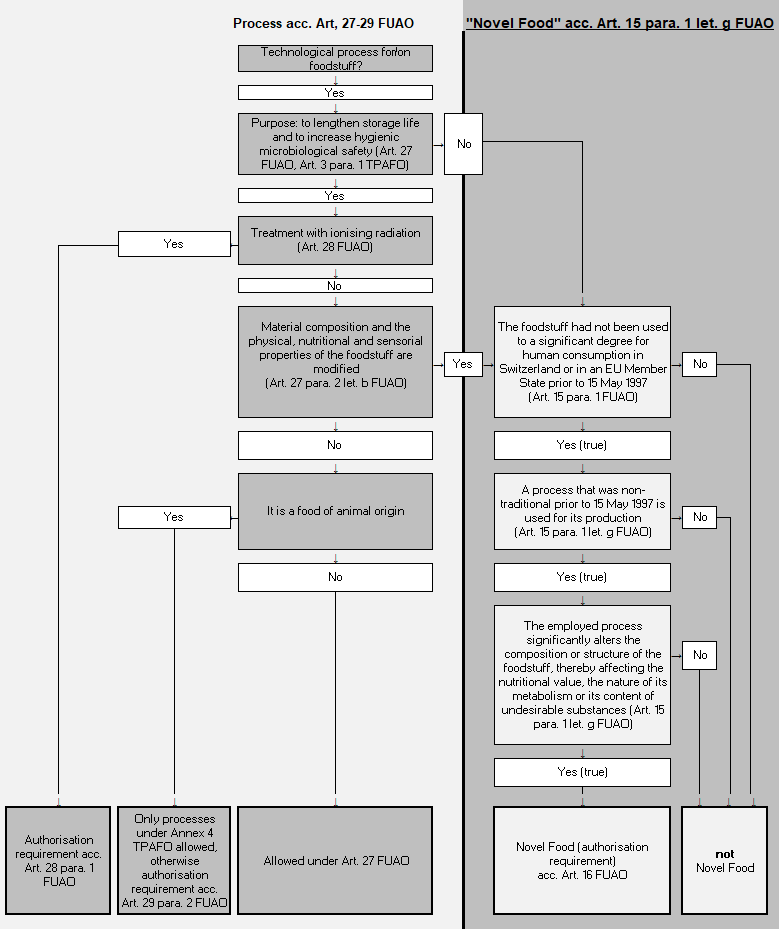
Foods fall under the Novel Food Provisions:
- when they had not been used to a significant degree for human consumption in Switzerland or in an EU Member State prior to 15 May 1997
- if they were produced using a process that was non-traditional prior to 15 May 1997
- if this process significantly altered their composition or structure, thereby affecting their nutritional value, the nature of their metabolism or their content of undesirable substances
These conditions are cumulative and have all to be fulfilled.
(Art. 15 para. 1 let. g of the Ordinance on Foodstuffs and Utility Articles; FUAO; SR 817.02) (in French).
Foods that do not fall under the Novel Food Provisions are those that have been treated with processes to lengthen the storage life and to increase hygienic microbiological safety (Art. 27 FUAO (in French), and Section 2 of the Ordinance on Technical Procedures and Technical Auxiliaries for the Treatment of Foodstuffs, TPAFO, SR 817. 022. 42) (in French). Only in the case when foodstuffs treated in this manner also result in significant modifications to their material composition and to their physical, nutritional or sensorial properties, is there also the requirement to examine the status as a Novel Food pursuant to Article 15 FUAO (in French).
In turn, the treatment of foodstuffs with ionising radiation always requires an authorisation in accordance with Art. 28 para. 1 FUAO (in French).
The following decision tree may also help to test whether a processed foodstuff is a Novel Food or not:
The classification test as a Novel Food, together with health protection, have always had to be carried out and documented in the scope of the obligation of self-regulation.
Classification examples:
Non-exhaustive examples of non-novelty are:
- Vegan alternatives to cheese, produced by fermenting non-novel vegetal foodstuffs with (non-novel) lactic acid bacteria typically used prior to 15 May 1997, and under the typical conditions used at that time (the technical designation of such products is determined in accordance with the information sheet 2020/3) (in French))
- Surface treatment of nuts with UV light in order to achieve hygienisation. If this treatment does not significantly modify the composition of the foodstuff in regard to its nutritional value, its metabolism or to the content of undesirable substances (process in accordance with Art. 27 FUAO) (in French)
- Foods that have been hygieneised with an HPP-process (high pressure processing). If this treatment does not significantly modify the composition of the foodstuff in regard to its nutritional value, its metabolism or to the content of undesirable substances (process in accordance with Art. 27 FUAO) (in French)
- If a foodstuff produced by a “novel” process, i.e. by a process that was not traditionally used prior to 15 May 1997, and which does not significantly modify the composition or structure of the foodstuff which would influence the nutritional value and its metabolism.
Non-exhaustive examples of novelty are:
- “UV-treated” foodstuffs with the objective of a targeted enrichment of vitamin D
- Foodstuffs that have been produced with a “novel” process, i.e. by a process that was not traditionally used prior to 15 May 1997. If this process significantly modifies the composition or structure of the foodstuff in regard to its nutritional value and its metabolism, and had not been used to a significant degree for human consumption (foodstuff) in Switzerland or in an EU Member State prior to 15 May 1997.
Labelling
Foods that have been treated with a process to lengthen the storage life and to increase hygienic microbiological safety shall be labelled in accordance with Annex 2 part A letters 1 and 3 of the FDHA Ordinance of 16 December 2016 in regard to the information on foodstuffs (FoodIO; SR 817.022.16) (in French).
Labelling of foodstuffs that are produced with “novel” processes and which are subject to authorisation as a Novel Food, will be defined in the scope of the authorisation procedure.
Last modification 10.07.2020